AI's First Blockbuster: Insilico Medicine's Rentosertib Advances to Final Clinical Trials
Insilico Medicine's Rentosertib is poised to become the first drug entirely discovered and designed by artificial intelligence to reach Phase 3 clinical trials—a watershed moment for AI-driven drug discovery that could reshape how the pharmaceutical industry develops treatments.
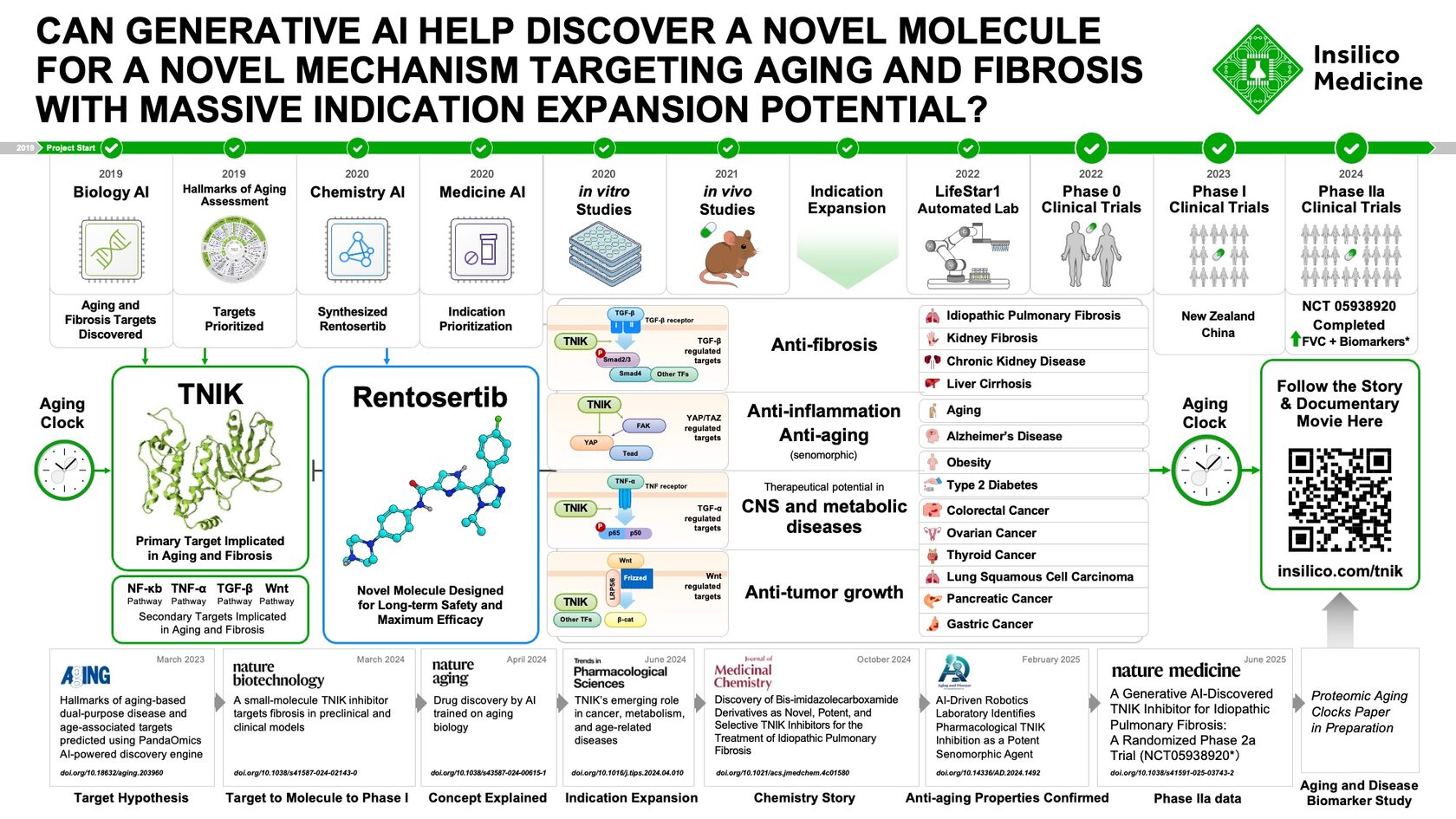
The AI Drug Discovery Inflection Point
The pharmaceutical industry's skepticism toward artificial intelligence-designed therapeutics is about to face a critical test. Insilico Medicine's Rentosertib is expected to enter Phase 3 clinical trials within the next 18 months, marking the first time a drug entirely discovered and designed using AI algorithms will advance to the final testing phase before regulatory approval. This milestone represents far more than a single company's achievement—it signals whether AI can genuinely accelerate drug discovery timelines and reduce development costs in an industry historically resistant to computational disruption.
What Makes Rentosertib Different
Rentosertib (ISM001-055) isn't simply a drug that used AI somewhere in its development pipeline. According to Insilico Medicine's own documentation, the compound was identified and optimized entirely through the company's proprietary generative AI platform—a claim that distinguishes it from thousands of other AI-assisted drug candidates still languishing in earlier trial phases.
The compound targets TNIK (Traf2 and Nck interacting kinase), a protein implicated in fibrosis and other degenerative conditions. Recent publication in Nature Medicine detailed Phase IIa results, though the company has not disclosed efficacy metrics that would justify the Phase 3 leap in public statements.
The Commercial Momentum Behind the Science
The timing of Rentosertib's advancement coincides with Insilico Medicine's Hong Kong IPO, which raised approximately $293 million, positioning the company as a major player in computational drug discovery. The IPO was characterized as the largest Hong Kong biotech listing of 2025, reflecting investor appetite for AI-driven pharmaceutical innovation.
However, this commercial success raises an important question: Is the market enthusiasm justified by the science, or are investors betting on the promise of AI drug discovery rather than proven results?
What Phase 3 Actually Means
Phase 3 trials represent the final regulatory gauntlet:
- Scale: Typically enrolls 1,000-5,000 patients across multiple sites
- Duration: Can span 2-3 years or longer
- Cost: Often exceeds $100 million per trial
- Failure rate: Approximately 50% of drugs entering Phase 3 fail to meet primary endpoints
The advancement to Phase 3 is significant, but it is not a guarantee of eventual FDA approval or commercial viability. Many drugs with promising Phase 2 data have stumbled at this stage.
The Broader Implications for AI in Pharma
If Rentosertib successfully completes Phase 3 and achieves regulatory approval, it would validate the core premise of AI-driven drug discovery: that machine learning can identify novel compounds and optimize them faster and more cost-effectively than traditional medicinal chemistry.
The pharmaceutical industry is watching closely. Insilico Medicine's platform claims to compress discovery timelines, but independent verification of these claims remains limited. Success with Rentosertib would likely accelerate investment in competing AI platforms and reshape how major pharma companies allocate R&D resources.
The Road Ahead
The next 18 months will be critical. Rentosertib's entry into Phase 3 represents a genuine inflection point—not because one drug is advancing, but because it will provide the first real-world evidence of whether AI-designed therapeutics can survive the rigorous scrutiny of late-stage clinical development. For an industry that has historically relied on serendipity and incremental optimization, this moment carries outsized significance.



